Structure of Metals
This page provides the chapters on the structure of metals from the "DOE Fundamentals Handbook: Material Science," DOE-HDBK-1017/1-93, U.S. Department of Energy, Jan 1993.
Other related chapters from the "DOE Fundamentals Handbook: Material Science" can be seen to the right.
- Structure of Metals
- Properties of Metals
- Material Selection
- Thermal Stress
- Brittle Fracture
Bonding
The arrangement of atoms in a material determines the behavior and properties of that material. Most of the materials used in the construction of a nuclear reactor facility are metals. In this chapter, we will discuss the various types of bonding that occurs in material selected for use in a reactor facility. The Chemistry Handbook discusses the bonding types in more detail.
Atomic Bonding
Matter, as we know it, exists in three common states. These three states are solid, liquid, and gas. The atomic or molecular interactions that occur within a substance determine its state. In this chapter, we will deal primarily with solids because solids are of the most concern in engineering applications of materials. Liquids and gases will be mentioned for comparative purposes only.
Solid matter is held together by forces originating between neighboring atoms or molecules. These forces arise because of differences in the electron clouds of atoms. In other words, the valence electrons, or those in the outer shell, of atoms determine their attraction for their neighbors. When physical attraction between molecules or atoms of a material is great, the material is held tightly together. Molecules in solids are bound tightly together. When the attractions are weaker, the substance may be in a liquid form and free to flow. Gases exhibit virtually no attractive forces between atoms or molecules, and their particles are free to move independently of each other.
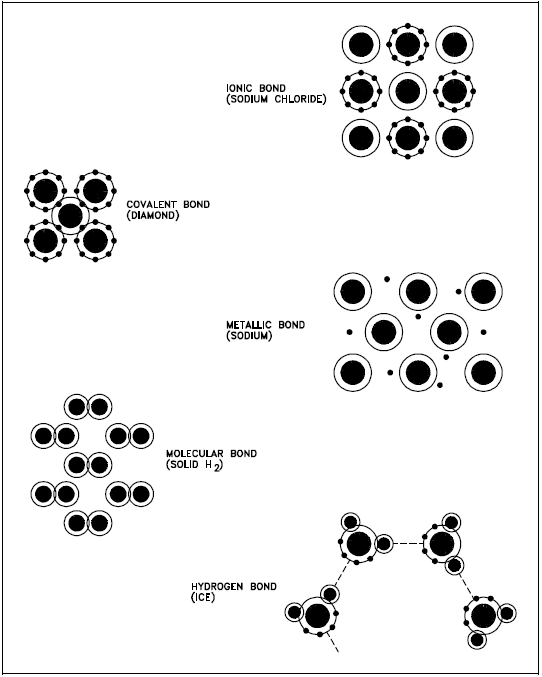
The types of bonds in a material are determined by the manner in which forces hold matter together. Figure 1 illustrates several types of bonds and their characteristics are listed below.
- Ionic bond - In this type of bond, one or more electrons are wholly transferred from an atom of one element to the atom of the other, and the elements are held together by the force of attraction due to the opposite polarity of the charge.
- Covalent bond - A bond formed by shared electrons. Electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with its neighbor. The electrons are then part of both atoms and both shells are filled.
- Metallic bond - In this type of bond, the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal, so that each electron can interact with many of the fixed atoms.
- Molecular bond - When the electrons of neutral atoms spend more time in one region of their orbit, a temporary weak charge will exist. The molecule will weakly attract other molecules. This is sometimes called the van der Waals or molecular bonds.
- Hydrogen bond - This bond is similar to the molecular bond and occurs due to the ease with which hydrogen atoms are willing to give up an electron to atoms of oxygen, fluorine, or nitrogen.
Some examples of materials and their bonds are identified in Table 1.
| Material | Bond |
|---|---|
| Sodium chloride | Ionic |
| Diamond | Covalent |
| Sodium | Metallic |
| Solid H2 | Molecular |
| Ice | Hydrogen |
The type of bond not only determines how well a material is held together, but also determines what microscopic properties the material possesses. Properties such as the ability to conduct heat or electrical current are determined by the freedom of movement of electrons. This is dependent on the type of bonding present. Knowledge of the microscopic structure of a material allows us to predict how that material will behave under certain conditions. Conversely, a material may be synthetically fabricated with a given microscopic structure to yield properties desirable for certain engineering applications.
Order in Microstructures
Solids have greater interatomic attractions than liquids and gases. However, there are wide variations in the properties of solid materials used for engineering purposes. The properties of materials depend on their interatomic bonds. These same bonds also dictate the space between the configuration of atoms in solids. All solids may be classified as either amorphous or crystalline.
Amorphous
Amorphous materials have no regular arrangement of their molecules. Materials like glass and paraffin are considered amorphous. Amorphous materials have the properties of solids. They have definite shape and volume and diffuse slowly. These materials also lack sharply defined melting points. In many respects, they resemble liquids that flow very slowly at room temperature.
Crystalline
In a crystalline structure, the atoms are arranged in a three-dimensional array called a lattice. The lattice has a regular repeating configuration in all directions. A group of particles from one part of a crystal has exactly the same geometric relationship as a group from any other part of the same crystal.
We have a number of structural calculators to choose from. Here are just a few:
Common Lattice Types
All metals used in a reactor have crystalline structures. Crystalline microstructures are arranged in three-dimensional arrays called lattices. This chapter will discuss the three most common lattice structures and their characteristics.
Common Crystal Structures
In metals, and in many other solids, the atoms are arranged in regular arrays called crystals. A crystal structure consists of atoms arranged in a pattern that repeats periodically in a three-dimensional geometric lattice. The forces of chemical bonding causes this repetition. It is this repeated pattern which control properties like strength, ductility, density (described in Module 2, Properties of Metals), conductivity (property of conducting or transmitting heat, electricity, etc.), and shape.
In general, the three most common basic crystal patterns associated with metals are: (a) the body-centered cubic, (b) the face-centered cubic, and (c) the hexagonal close-packed. Figure 2 shows these three patterns.
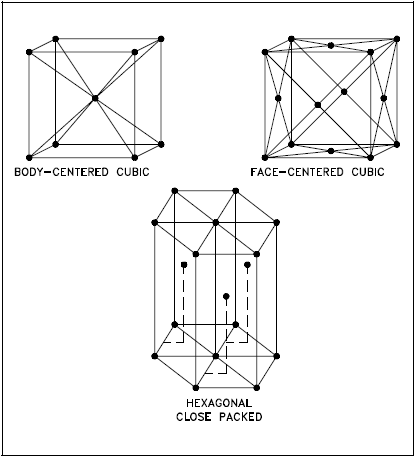
Body-centered Cubic
In a body-centered cubic (BCC) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the body center of the cube.
Face-centered Cubic
In a face-centered cubic (FCC) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the center of each of the faces of the cube.
Hexagonal Close-packed
In a hexagonal close-packed (HCP) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed.
Most diagrams of the structural cells for the BCC and FCC forms of iron are drawn as though they are of the same size, as shown in Figure 2, but they are not. In the BCC arrangement, the structural cell, which uses only nine atoms, is much smaller.
Metals such as α-iron (Fe) (ferrite), chromium (Cr), vanadium (V), molybdenum (Mo), and tungsten (W) possess BCC structures. These BCC metals have two properties in common, high strength and low ductility (which permits permanent deformation). FCC metals such as γ-iron (Fe) (austenite), aluminum (Al), copper (Cu), lead (Pb), silver (Ag), gold (Au), nickel (Ni), platinum (Pt), and thorium (Th) are, in general, of lower strength and higher ductility than BCC metals. HCP structures are found in beryllium (Be), magnesium (Mg), zinc (Zn), cadmium (Cd), cobalt (Co), thallium (Tl), and zirconium (Zr).
Grain Structure and Boundary
Metals contain grains and crystal structures. The individual needs a microscope to see the grains and crystal structures. Grains and grain boundaries help determine the properties of a material.
Grain Structure and Boundary
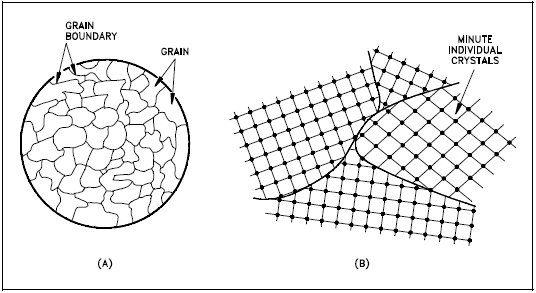
If you were to take a small section of a common metal and examine it under a microscope, you would see a structure similar to that shown in Figure 3(a). Each of the light areas is called a grain, or crystal, which is the region of space occupied by a continuous crystal lattice. The dark lines surrounding the grains are grain boundaries. The grain structure refers to the arrangement of the grains in a metal, with a grain having a particular crystal structure.
The grain boundary refers to the outside area of a grain that separates it from the other grains. The grain boundary is a region of misfit between the grains and is usually one to three atom diameters wide. The grain boundaries separate variously-oriented crystal regions (polycrystalline) in which the crystal structures are identical. Figure 3(b) represents four grains of different orientation and the grain boundaries that arise at the interfaces between the grains.
A very important feature of a metal is the average size of the grain. The size of the grain determines the properties of the metal. For example, smaller grain size increases tensile strength and tends to increase ductility. A larger grain size is preferred for improved high-temperature creep properties. Creep is the permanent deformation that increases with time under constant load or stress. Creep becomes progressively easier with increasing temperature. Stress and strain are covered in Module 2, Properties of Metals, and creep is covered in Module 5, Plant Materials.
(a) Microscopic (b) Atomic
Another important property of the grains is their orientation. Figure 4(a) represents a random arrangement of the grains such that no one direction within the grains is aligned with the external boundaries of the metal sample. This random orientation can be obtained by cross rolling the material. If such a sample were rolled sufficiently in one direction, it might develop a grain-oriented structure in the rolling direction as shown in Figure 4(b). This is called preferred orientation. In many cases, preferred orientation is very desirable, but in other instances, it can be most harmful. For example, preferred orientation in uranium fuel elements can result in catastrophic changes in dimensions during use in a nuclear reactor.
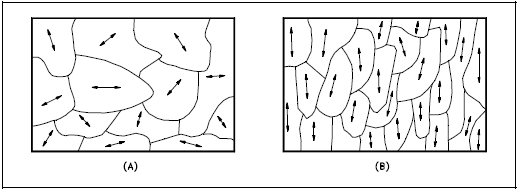
(a) Random (b) Preferred
We have a number of structural calculators to choose from. Here are just a few:
Polymorphism
Metals are capable of existing in more than one form at a time. This chapter will discuss this property of metals.
Polymorphism Phases
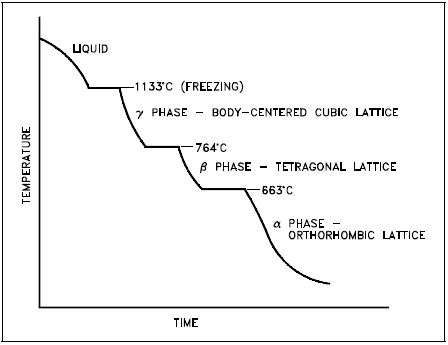
Polymorphism is the property or ability of a metal to exist in two or more crystalline forms depending upon temperature and composition. Most metals and metal alloys exhibit this property. Uranium is a good example of a metal that exhibits polymorphism. Uranium metal can exist in three different crystalline structures. Each structure exists at a specific phase, as illustrated in Figure 5.
- The alpha phase, from room temperature to 663°C
- The beta phase, from 663°C to 764°C
- The gamma phase, from 764°C to its melting point of 1133°C
Alpha Phase
The alpha (α) phase is stable at room temperature and has a crystal system characterized by three unequal axes at right angles.
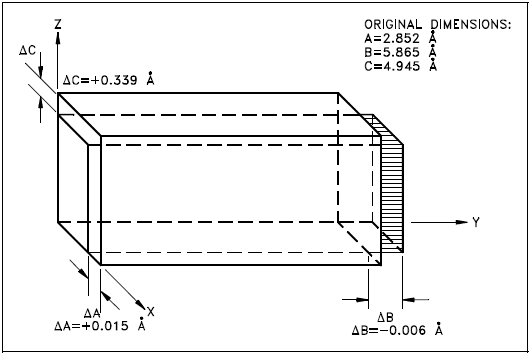
In the alpha phase, the properties of the lattice are different in the X, Y, and Z axes. This is because of the regular recurring state of the atoms is different. Because of this condition, when heated the phase expands in the X and Z directions and shrinks in the Y direction. Figure 6 shows what happens to the dimensions (Å = angstrom, one hundred-millionth of a centimeter) of a unit cell of alpha uranium upon being heated.
As shown, heating and cooling of alpha phase uranium can lead to drastic dimensional changes and gross distortions of the metal. Thus, pure uranium is not used as a fuel, but only in alloys or compounds.
Beta Phase
The beta (β) phase of uranium occurs at elevated temperatures. This phase has a tetragonal (having four angles and four sides) lattice structure and is quite complex.
Gamma Phase
The gamma (γ) phase of uranium is formed at temperatures above those required for beta phase stability. In the gamma phase, the lattice structure is BCC and expands equally in all directions when heated.
Additional Examples
Two additional examples of polymorphism are listed below.
- Heating iron to 907°C causes a change from BCC (alpha, ferrite) iron to the FCC (gamma, austenite) form.
- Zirconium is HCP (alpha) up to 863°C, where it transforms to the BCC (beta, zirconium) form.
The properties of one polymorphic form of the same metal will differ from those of another polymorphic form. For example, gamma iron can dissolve up to 1.7% carbon, whereas alpha iron can dissolve only 0.03%.
Alloys
Most of the materials used in structural engineering or component fabrication are metals. Alloying is a common practice because metallic bonds allow joining of different types of metals.
Alloys
An alloy is a mixture of two or more materials, at least one of which is a metal. Alloys can have a microstructure consisting of solid solutions, where secondary atoms are introduced as substitutionals or interstitials (discussed further in the next chapter and Module 5, Plant Materials) in a crystal lattice. An alloy might also be a crystal with a metallic compound at each lattice point. In addition, alloys may be composed of secondary crystals imbedded in a primary polycrystalline matrix. This type of alloy is called a composite (although the term "composite" does not necessarily imply that the component materials are metals). Module 2, Properties of Metals, discusses how different elements change the physical properties of a metal.
Common Characteristics of Alloys
Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. Strength is the most important criterion by which many structural materials are judged. Therefore, alloys are used for engineering construction. Steel, probably the most common structural metal, is a good example of an alloy. It is an alloy of iron and carbon, with other elements to give it certain desirable properties.
As mentioned in the previous chapter, it is sometimes possible for a material to be composed of several solid phases. The strengths of these materials are enhanced by allowing a solid structure to become a form composed of two interspersed phases. When the material in question is an alloy, it is possible to quench (discussed in more detail in Module 2, Properties of Metals) the metal from a molten state to form the interspersed phases. The type and rate of quenching determines the final solid structure and, therefore, its properties.
Type 304 Stainless Steel
Type 304 stainless steel (containing 18%-20% chromium and 8%-10.5% nickel) is used in the tritium production reactor tanks, process water piping, and original process heat exchangers. This alloy resists most types of corrosion.
Composition of Common Engineering Materials
The wide variety of structures, systems, and components found in DOE nuclear facilities are made from many different types of materials. Many of the materials are alloys with a base metal of iron, nickel, or zirconium. The selection of a material for a specific application is based on many factors including the temperature and pressure that the material will be exposed to, the materials resistance to specific types of corrosion, the materials toughness and hardness, and other material properties.
One material that has wide application in the systems of DOE facilities is stainless steel. There are nearly 40 standard types of stainless steel and many other specialized types under various trade names. Through the modification of the kinds and quantities of alloying elements, the steel can be adapted to specific applications. Stainless steels are classified as austenitic or ferritic based on their lattice structure. Austenitic stainless steels, including 304 and 316, have a face-centered cubic structure of iron atoms with the carbon in interstitial solid solution. Ferritic stainless steels, including type 405, have a body-centered cubic iron lattice and contain no nickel. Ferritic steels are easier to weld and fabricate and are less susceptible to stress corrosion cracking than austenitic stainless steels. They have only moderate resistance to other types of chemical attack.
Other metals that have specific applications in some DOE nuclear facilities are inconel and zircaloy. The composition of these metals and various types of stainless steel are listed in Table 2 below.
| %Fe | %C Max |
%Cr | %Ni | %Mo | %Mn Max |
%Si Max |
%Zr | |
|---|---|---|---|---|---|---|---|---|
| 304 Stainless Steel | Bal. | 0.08 | 19 | 10 | 2 | 1 | ||
| 304L Stainless Steel | Bal. | 0.03 | 18 | 8 | 2 | 1 | ||
| 316 Stainless Steel | Bal. | 0.08 | 17 | 12 | 2.5 | 2 | 1 | |
| 316L Stainless Steel | Bal. | 0.03 | 17 | 12 | 2.5 | 2 | ||
| 405 Stainless Steel | Bal. | 0.08 | 13 | 1 | 1 | |||
| Inconel | 8 | 0.15 | 15 | Bal. | 1 | 0.5 | ||
| Zircaloy-4 | 0.21 | 0.1 | Bal. |
We have a number of structural calculators to choose from. Here are just a few:
Imperfections in Metals
The discussion of order in microstructures in the previous chapters assumed idealized microstructures. In reality, materials are not composed of perfect crystals, nor are they free of impurities that alter their properties. Even amorphous solids have imperfections and impurities that change their structure.
Microscopic Imperfections
Microscopic imperfections are generally classified as either point, line, or interfacial imperfections.
- Point imperfections have atomic dimensions.
- Line imperfections or dislocations are generally many atoms in length.
- Interfacial imperfections are larger than line defects and occur over a two-dimensional area.
Point Imperfections
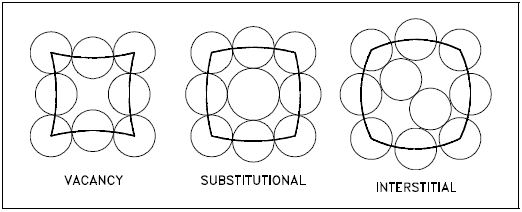
Point imperfections in crystals can be divided into three main defect categories. They are illustrated in Figure 7.
- Vacancy defects result from a missing atom in a lattice position. The vacancy type of defect can result from imperfect packing during the crystallization process, or it may be due to increased thermal vibrations of the atoms brought about by elevated temperature.
- Substitutional defects result from an impurity present at a lattice position.
-
Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of being at its lattice position. Interstitial refers to locations between atoms in a lattice structure.Interstitial impurities called network modifiers act as point defects in amorphous solids. The presence of point defects can enhance or lessen the value of a material for engineering construction depending upon the intended use.
Line Imperfections
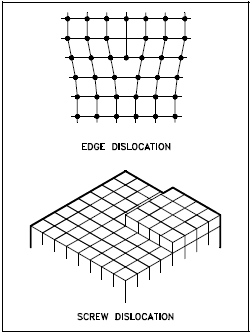
Line imperfections are called dislocations and occur in crystalline materials only. Dislocations can be an edge type, screw type, or mixed type, depending on how they distort the lattice, as shown in Figure 8. It is important to note that dislocations cannot end inside a crystal. They must end at a crystal edge or other dislocation, or they must close back on themselves.
Edge dislocations consist of an extra row or plane of atoms in the crystal structure. The imperfection may extend in a straight line all the way through the crystal or it may follow an irregular path. It may also be short, extending only a small distance into the crystal causing a slip of one atomic distance along the glide plane (direction the edge imperfection is moving).
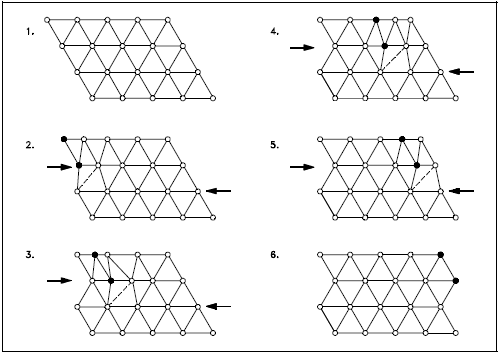
The slip occurs when the crystal is subjected to a stress, and the dislocation moves through the crystal until it reaches the edge or is arrested by another dislocation, as shown in Figure 9. Position 1 shows a normal crystal structure. Position 2 shows a force applied from the left side and a counterforce applied from the right side. Positions 3 to 5 show how the structure is slipping. Position 6 shows the final deformed crystal structure. The slip of one active plane is ordinarily on the order of 1000 atomic distances and, to produce yielding, slip on many planes is required.
Screw dislocations can be produced by a tearing of the crystal parallel to the slip direction. If a screw dislocation is followed all the way around a complete circuit, it would show a slip pattern similar to that of a screw thread. The pattern may be either left or right handed. This requires that some of the atomic bonds are re-formed continuously so that the crystal has almost the same form after yielding that it had before.
The orientation of dislocations may vary from pure edge to pure screw. At some intermediate point, they may possess both edge and screw characteristics. The importance of dislocations is based on the ease at which they can move through crystals.
Interfacial Imperfections
Interfacial imperfections exist at an angle between any two faces of a crystal or crystal form. These imperfections are found at free surfaces, domain boundaries, grain boundaries, or interphase boundaries. Free surfaces are interfaces between gases and solids. Domain boundaries refer to interfaces where electronic structures are different on either side causing each side to act differently although the same atomic arrangement exists on both sides. Grain boundaries exist between crystals of similar lattice structure that possess different spatial orientations. Polycrystalline materials are made up of many grains which are separated by distances typically of several atomic diameters. Finally, interphase boundaries exist between the regions where materials exist in different phases (i.e., BCC next to FCC structures).
Macroscopic Defects
Three-dimensional macroscopic defects are called bulk defects. They generally occur on a much larger scale than the microscopic defects. These macroscopic defects generally are introduced into a material during refinement from its raw state or during fabrication processes.
The most common bulk defect arises from foreign particles being included in the prime material. These second-phase particles, called inclusions, are seldom wanted because they significantly alter the structural properties. An example of an inclusion may be oxide particles in a pure metal or a bit of clay in a glass structure.
Other bulk defects include gas pockets or shrinking cavities found generally in castings. These spaces weaken the material and are therefore guarded against during fabrication. The working and forging of metals can cause cracks that act as stress concentrators and weaken the material. Any welding or joining defects may also be classified as bulk defects.
PDH Classroom offers a continuing education course based on this structure of metals reference page. This course can be used to fulfill PDH credit requirements for maintaining your PE license.
Now that you've read this reference page, earn credit for it!